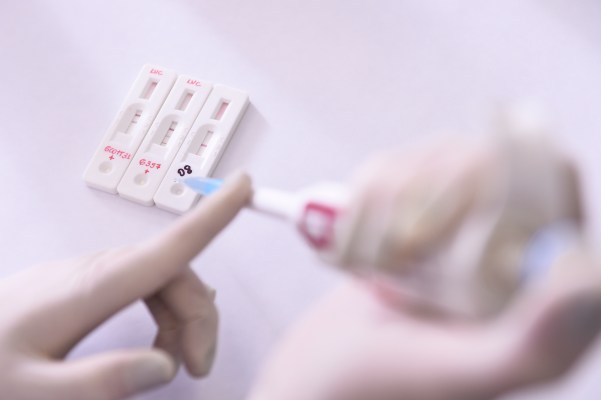
The FDA just okayed multiple 15-minute blood tests to screen for coronavirus, but there are caveats
1:08pm PDT - March 27th, 2020 covid-19 - TechCrunchOn Thursday, the FDA amended their emergency policy around diagnostic testing for SARS-CoV-2, the novel coronavirus that causes COVID-19. Following on a change made March 16, the agency opened the door for a number of specific private entities and labs to dev…
